
Dec 10, 2017
 The Working Conference and General Assembly of the Stroke Alliance for Europe (SAFE) took place at the Westin Hotel, Zagreb from 6-8th December, 2017. This was the most successful SAFE annual conference to date, with around one hundred delegates from more than 30 European countries.
The Working Conference and General Assembly of the Stroke Alliance for Europe (SAFE) took place at the Westin Hotel, Zagreb from 6-8th December, 2017. This was the most successful SAFE annual conference to date, with around one hundred delegates from more than 30 European countries.
SAFE delegates have approved at the General Assembly two new applications for membership that have been previously approved by the board. These are from the Irish Heart Foundation and Beyinder Turkey.
A stroke support organisation Sdružení pro rehabilitaci osob po cévní mozkové příhodě (Czech Republic) have been reinstatement as a SAFE member.
France AVC did not comply with membership requirements during the 2017 and therefore did not become a full member until now. At this General Assembly, they have submitted the missing documents and the delegates confirmed their membership. (more…)
Dec 4, 2017
Please read below an excerpt from an article published in Croatian online portal www.24sata.hr about the SAFE Working Conference 2017.
Although the number of deaths caused by stroke is in decline, there are more people who survive an acute stroke, but there are also others who remain with a permanent disability…
Stroke Alliance for Europe (SAFE) is organising an Annual Working Conference in Zagreb, Croatia, from 6-8th December 2017. There will be more then 100 delegates from over 30 European countries. The Working Conference will cover topics such as current and future projects aiming to reduce the number of new strokes in Europe and enable better life for stroke survivors and their families with the consequences of this disease.
SAFE started a project called „Stroke Support Organisation Faculty Tool- SSOFT“. At the moment, the focus is on building an online platform for education of stroke support organisations and individuals about stroke and how to advocate for improvement of the prevention, treatment and post-stroke care.
In 2017, SAFE has finished a big project “Burden of Stroke in Europe”, in which Croatia took an active part.
Please read the whole article here.

Dec 1, 2017
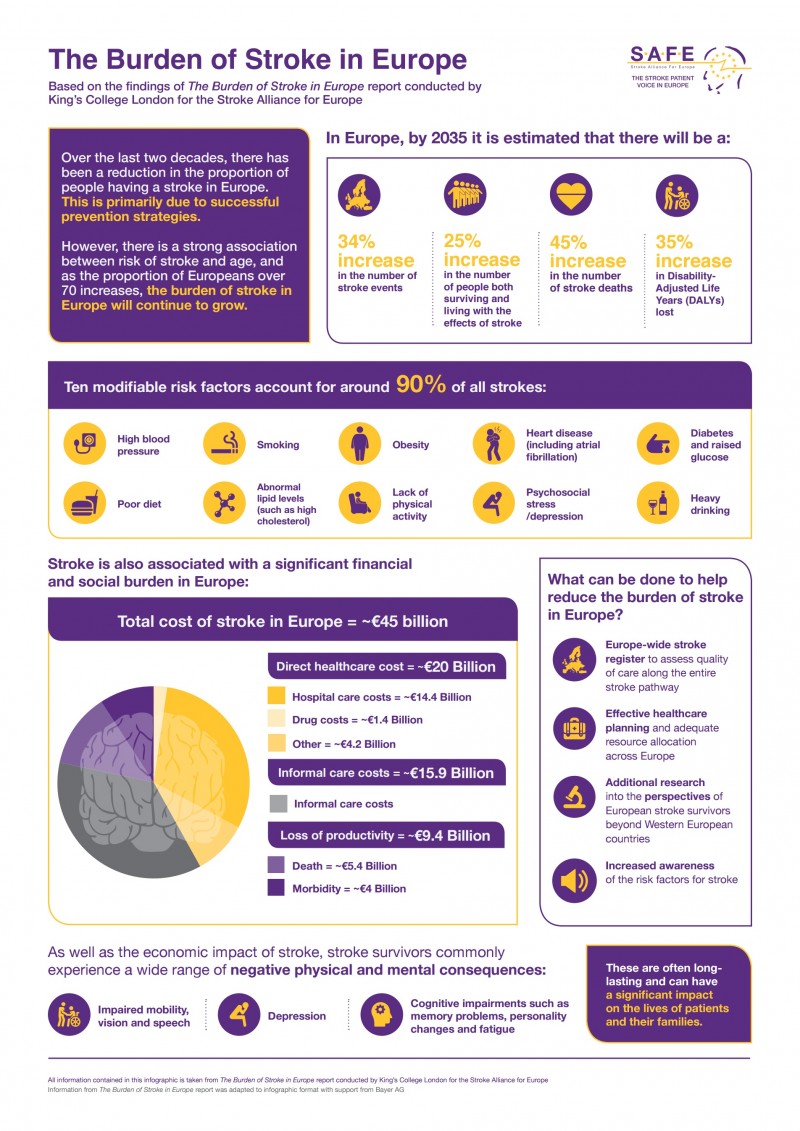
With grateful thanks for its generosity to our industry partner Bayer, we are now presenting you with infographics based on the data from the Burden of Stroke Report, in SAFE branding, showing data country by country.
Please find your country infographic below these two general infographics, showing the size of the burden and the state of the stroke treatment across Europe.


You can open and download any specific infographic by clicking on the name of the country from the list below.
Austria
Belgium
Bulgaria
Croatia
Cyprus
Czech Republic
Denmark
Estonia
Finland
France
Germany
Greece
Hungary
Iceland
Ireland
Israel
Italy
Latvia
Lithuania
Luxembourg
Macedonia
Malta
Netherlands
Norway
Poland
Portugal
Romania
Serbia
Slovakia
Slovenia
Spain
Sweden
UK
Ukraine

Nov 29, 2017
All countries face the challenge of funding ever-new and expensive treatments, with increasing pressure on budgets, harmful deficit spending, and ever-higher health insurance premiums. One solution to these concerns is to scale back on outdated or low-value procedures; not unlike clearing out one’s clothing closet to make room for more contemporary wear.
Yet there is little understanding of “exnovation,” or the scaling-back of expensive medical treatments for certain medical practices. To better understand this process, researchers at The Dartmouth Institute for Health Policy and Clinical Practice studied nearly 10,000 physicians who performed carotid revascularization — a surgical procedure used to reduce the risk of stroke by correcting narrowing in the carotid artery — on elderly Medicare patients between 2006-2013. Its use is increasingly controversial, particularly among older patients and for those who aren’t exhibiting symptoms of carotid artery narrowing. (more…)

Nov 27, 2017
WAKE-UP is a European multicentre investigator-initiated randomized placebo-controlled clinical trial of MRI based thrombolysis in acute stroke patients with unknown time of symptom onset, e.g. due to recognition of stroke symptoms on awakening.
Stroke is a devastating disease leading to death and disability in large numbers of patients with massive social and economic impact. Intravenous thrombolysis with Alteplase is available as effective and safe treatment of acute stroke within 4.5 hours of symptom onset. However, in about 20% of acute stroke patients time of symptom onset is unknown e.g. because symptoms are recognized when waking-up from sleep in the morning. This large group of patients is currently excluded from treatment with Alteplase only due to the missing information on the time of symptom onset. In preparatory work the WAKE-UP consortium has developed an innovative approach of using brain MRI as surrogate marker of stroke lesion age which may be used to identify stroke patients likely to benefit from thrombolysis.
The Project started in 2013. As of June 30, enrollment in the WAKE-UP trial was stopped following a decision by the Steering Committee. By the end of enrollment, 1,377 patients were enrolled in WAKE-UP with 501 patients randomized and 876 screen failures. The final meeting was held in Hamburg, Germany, in November 2017.
 WAKE UP was led by an active and motivated consortium combining central trial management with decentralised organisation with national coordinators, with a clear communication structure and share of responsibilities, which led to the successful completion of the project.
WAKE UP was led by an active and motivated consortium combining central trial management with decentralised organisation with national coordinators, with a clear communication structure and share of responsibilities, which led to the successful completion of the project.
WAKE-UP is a European collaborative research project launched by a consortium of academic and SME partners destined to improve the treatment of stroke patients. The core of WAKE-UP is an investigator-initiated randomized controlled trial of MRI based thrombolysis in patients waking up with stroke symptoms.
The objective of WAKE-UP is to test efficacy and safety of MRI-based intravenous thrombolysis with Alteplase in patients waking up with stroke symptoms or patients with unknown symptom onset. By this, WAKE-UP aims at providing a new safe and effective treatment option for acute stroke patients waking up with stroke symptoms.
The project is centrally coordinated by the Universitätsklinikum Hamburg-Eppendorf (Prof. Christian Gerloff; deputy coordinator: Dr. Götz Thomalla).
During the WAKE UP trial, imaging software (SONIA) was developed for the purpose of this project, yet this software can also be used even when WAKE UP trial is done, for other research projects in the future.
One of the collateral benefits of this project was that clinicians/ investigators were trained in reading MRI images, providing a learning opportunity for these stroke professionals in the interpretation of MRI in acute stroke diagnostics. Almost 400 investigators from eight European countries participated in a structured software-based training.
WAKE UP trial proved to be a good example on how to involve patients who are unable to give consent.
Repeated interim analysis of safety data from the trial and evaluation by the independent Data and Safety Monitoring Board already showed that the intervention performed in this trial was proven to be safe for patients.
The final results of the main research will be presented to the public in May 2018 at ESOC 2018 conference.
For further information, please visit the Wake-Up project’s web site.
WAKE-UP – The work leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 278276.
Please see or download WAKE-UP update in English language or in languages from the following countries:
Iceland
Hungary
Croatia
Serbia
Belgium
Germany
Slovenia
Greece
Cyprus
Spain
Georgia
Luxembourg
Israel
Netherlands

Nov 27, 2017
by ESO | 24.11.2017
Author: Marialuisa Zedde, MD, Arcispedale Santa Maria Nuova IRCCS, Reggio Emilia, Italy
ESO organized this workshop in Munich on November 16 2017 just before the Garmish conference.
The aim of this workshop was to detail methodological issues in guidelines production, mainly following step by step the GRADE methodology.
Both speakers and talks in the workshop were of high quality level and each talk was complete and interactive. The ability of the speakers was to make simple and easy to listen and learn some complex topics, being open to the discussion and to answer the questions from the audience. (more…)

 The Working Conference and General Assembly of the Stroke Alliance for Europe (SAFE) took place at the Westin Hotel, Zagreb from 6-8th December, 2017. This was the most successful SAFE annual conference to date, with around one hundred delegates from more than 30 European countries.
The Working Conference and General Assembly of the Stroke Alliance for Europe (SAFE) took place at the Westin Hotel, Zagreb from 6-8th December, 2017. This was the most successful SAFE annual conference to date, with around one hundred delegates from more than 30 European countries.





 WAKE UP was led by an active and motivated consortium combining central trial management with decentralised organisation with national coordinators, with a clear communication structure and share of responsibilities, which led to the successful completion of the project.
WAKE UP was led by an active and motivated consortium combining central trial management with decentralised organisation with national coordinators, with a clear communication structure and share of responsibilities, which led to the successful completion of the project.



