Oxygen after stroke – the PROOF trial
Can high-dose oxygen therapy reduce the effects of stroke? A new international study called PROOF, funded by the EU, will investigate.
An ischaemic stroke is the most common type of stroke. The arteries that supply the brain with blood become clogged by clots. This kills the cells at the centre of the stroke and puts those nearby at great risk.

The longer the brain suffers from oxygen and blood deficiency, the more serious the consequences, and the more brain tissue will die. Emergency treatment must therefore get rid of the blocked arteries as quickly as possible.
Starting January 2017 at the Tübingen University Clinic – and at eleven other clinical centres in eight European countries – the PROOF trial will test whether the consequences of stroke can be reduced by rapidly applying high-dose oxygen therapy to new patients.
The high-dose oxygen therapy is very simple and low-cost. The patient, after having been diagnosed with an ischemic stroke after their scan, wears a mask through which they inhale almost pure oxygen at a flow of 40 litres per minute. This keeps the oxygen content in the blood as high as it can be.
The aim is to ensure that the surrounding brain tissue at risk, which will have less oxygen but is not yet dead, is stabilized by the high oxygen content coming in. This continues
until the clot is removed and the blood circulation is improved again. The treatment therefore aims at the so-called “shadow zones” of the stroke, sometimes called the “rescue zones”. These may include the entire stroke area in some patients.
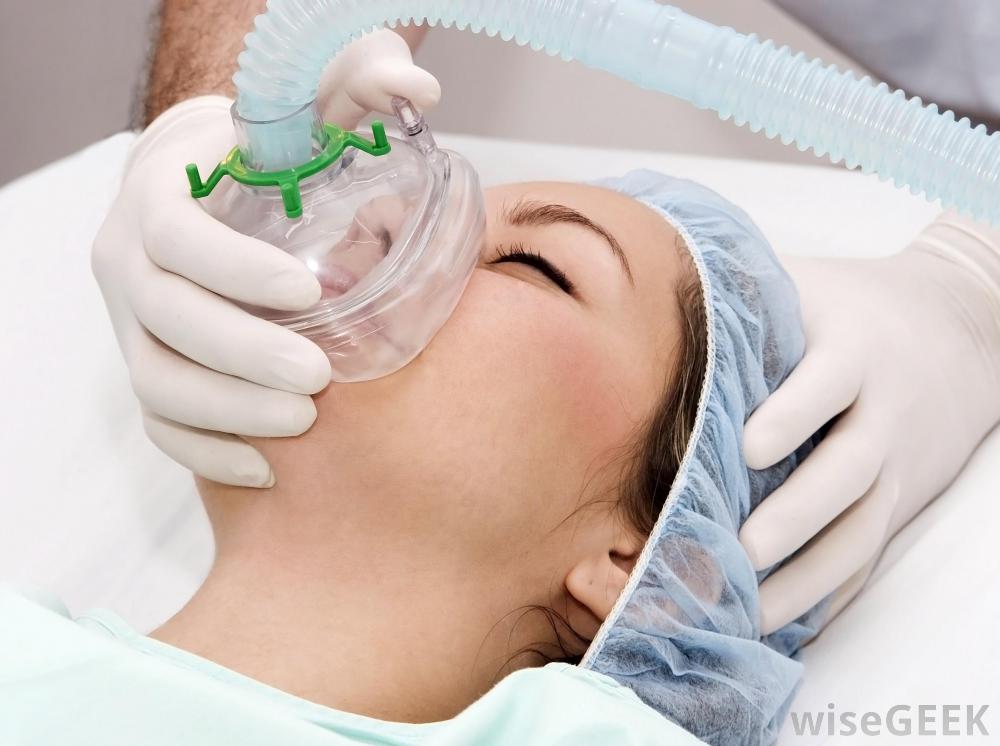
Image: wiseGeek
PROOF is the first trial to thoroughly investigate high-dose oxygenation in stroke patients. If it proves that the brain can be saved by this, it could also bring great benefits to patients who are not near a clinic where stroke can be treated and who have longer access times to emergency care.
The study is coordinated by Dr. Sven Poli, Senior Physician of the Department of Neurology with focus on neurovascular diseases at the University Hospital Tübingen. The European-wide study is funded by the European Union with around six million euros.
SAFE will work on communication of information about the PROOF trial to non-clinical audiences. For further detail please contact research@safestroke.eu




